Search Results
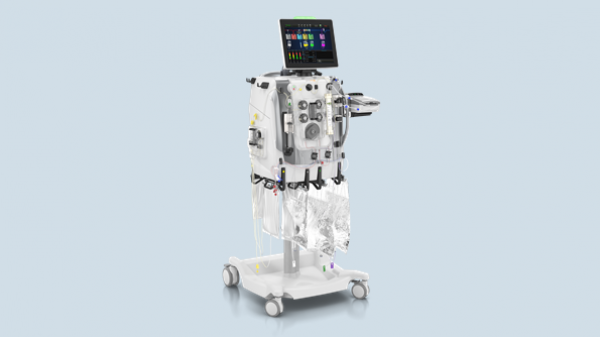
Baxter Receives CE Mark for PrisMax, the Next-Generation System for Continuous Renal Replacement and Organ Support Therapies
Baxter International Inc. (NYSE:BAX), a global leader in acute care, announced today the company received CE mark of both its PrisMax system and its TherMax blood warmer. PrisMax is the company’s next-generation technology for continuous renal replacement and organ support therapies.
Baxter Highlights Clinical Data at ASN: Kidney Week Showing How New Technologies are Advancing Dialysis Care Across Modalities
Baxter International Inc. (NYSE:BAX), a global innovator in renal care, highlighted findings from HDx (expanded hemodialysis) and peritoneal dialysis (PD) studies presented at the American Society of Nephrology (ASN): Kidney Week, Oct. 23-28, showing how novel renal care technologies are positively impacting patient care and clinic efficiency.
Baxter Announces U.S. Regulatory Filing for PrisMax Acute Care System and Presentation of 15 Abstracts to Start ASN: Kidney Week 2018
Baxter International Inc. (NYSE:BAX), a leading global medical products company, announced it has submitted the PrisMax system for 510(k) clearance to the U.S. Food and Drug Administration (FDA).
Baxter to Showcase Critical Care Innovation at the 2018 European Society of Intensive Care Medicine Congress
Baxter International Inc. (NYSE:BAX), a leading global medical products company, will highlight its latest innovations in critical care at the 2018 European Society of Intensive Care Medicine (ESICM) congress in Paris from Oct. 20-24.
Baxter and ASPEN Launch 'Smart PN' Tools to Reinforce Appropriate Use of Parenteral Nutrition
Today the American Society for Parenteral and Enteral Nutrition (ASPEN), an organization which drives the science and practice of clinical nutrition, and Baxter International Inc. (NYSE: BAX), a global leader in clinical nutrition, launched an educational video series on the appropriate use of parenteral nutrition (PN), as part of the organizations’ “SmartPN” collaboration to help reduce clinical malnutrition that was announced last year.
Baxter to Present at the 27th Annual Credit Suisse Healthcare Conference
Baxter International Inc. (NYSE:BAX), a leading global medical products company, will present at the 27th Annual Credit Suisse Healthcare Conference in Scottsdale, Ariz. Jay Saccaro, Baxter’s chief financial officer, is scheduled to present on Wednesday, November 14, 2018 at 8:00 a.m. Mountain Time.
Baxter and Mayo Clinic Announce Collaboration to Establish Renal Care Center of Excellence in the U.S.
Baxter International Inc. (NYSE:BAX), a global innovator in renal care, and Mayo Clinic today announced a new collaboration to develop a renal care center of excellence in the U.S. The center will serve patients across the continuum of renal care — from chronic kidney disease (CKD) management through transplant — and drive better patient outcomes.
Baxter Collaborates with University of Southern California Center for Body Computing to Advance Digital Health Solutions
Baxter International Inc. (NYSE: BAX), a leading global medical products company, announced today a collaboration with the University of Southern California (USC) Center for Body Computing (CBC) to research and develop innovative digital solutions designed to help save and sustain lives.
New Data on Floseal Surgical Hemostat Quantifies Clinical and Healthcare Resource Outcomes in Spine Surgery Cases
Baxter International Inc. (NYSE:BAX), a global leader in advancing surgical innovation, announced the publication of two new analyses of real-world data regarding the use of Floseal Hemostatic Matrix in spinal surgeries. The retrospective studies observed better clinical and resource use outcomes in cases with billed charges for Floseal, when compared to cases with charges for Floseal in addition to certain non-flowable hemostatic agents.
Baxter Announces U.S. FDA Clearance of Altapore Bioactive Bone Graft in Posterolateral Spine Surgery
Baxter International Inc. (NYSE:BAX), a global leader in advancing surgical innovation, today announced U.S. Food and Drug Administration (FDA) clearance of ALTAPORE Bioactive Bone Graft, a next-generation bioactive and osteoconductive bone graft substitute, for use as an autograft extender in posterolateral spinal fusion. ALTAPORE had previously been cleared for use in orthopedic surgical procedures in the extremities and pelvis.