Baxter Launches Progressa+ Next Gen ICU Bed to Help Address Complex Critical Care Needs
Developed in collaboration with nurses and therapists to help ease the burden on critical care teams
Supports care teams as they address pulmonary, skin and mobility challenges common in the ICU environment
Launches in U.S. with global expansion planned to begin later this year
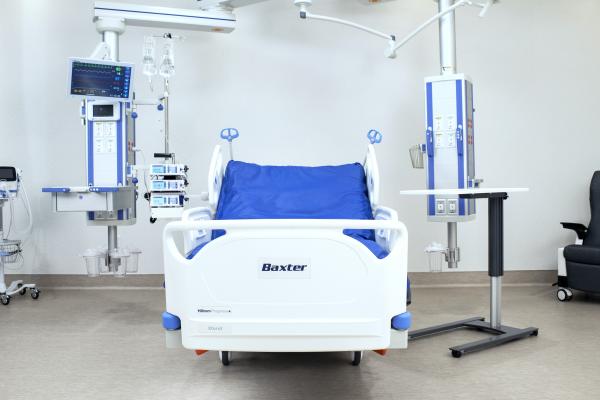
Progressa+ ICU bed
DEERFIELD, Ill., -
Baxter International Inc. (NYSE:BAX), an innovator in connected beds for med-surg and the intensive care unit (ICU), announced today the U.S. launch of its new Hillrom Progressa+ bed for the ICU. Progressa+ offers new technology and features that help make it easier for nurses to care for patients, while supporting patient recovery.
“Our new and improved Progressa+ is a ‘true ICU bed,’ designed for the realities of critical care, where patient needs are high and clinical staff can be stretched thin,” said Julie Brewer, president, Baxter’s Patient Support Systems, Global Surgical Solutions and Care Communications businesses. "We asked ICU care teams how we could make their jobs easier, and then designed the next generation of our leading ICU bed with features that help address challenges, such as reducing strain on nursing resources, lessening risk of pressure injuries and simplifying patient positioning."
Progressa+ includes technologies designed to help support pulmonary needs, protect skin and support early mobility protocols.[i]
- Provide pulmonary support: Progressa+ provides in-bed percussion, vibration and continual lateral rotation therapies designed to help reduce pulmonary complications associated with immobility. In addition, the improved bed frame provides easier access to the head of the bed for intubation and other procedures.
- Protect skin: Progressa+ features enhanced support surfaces designed to help support optimal skin protection and wound healing. A new top cover allows for easier cleaning.
- Support early patient mobility: Used in conjunction with an integrated lift system, Progressa+ helps the care team support patient mobility protocols while minimizing the risk of clinician injury. One-button FullChair with sit-to-stand Chair Egress helps clinicians move patients safely and easily.
Progressa+ is now available in the United States. Baxter plans to launch its latest ICU bed in additional markets across the globe throughout the next 18 months. Progressa+ is part of Baxter’s comprehensive portfolio of beds and surfaces that are tailored for nearly all care environments.
About Baxter
Every day, millions of patients, caregivers and healthcare providers rely on Baxter’s leading portfolio of diagnostic, critical care, kidney care, nutrition, hospital and surgical products used across patient homes, hospitals, physician offices and other sites of care. For more than 90 years, we’ve been operating at the critical intersection where innovations that save and sustain lives meet the healthcare providers who make it happen. With products, digital health solutions and therapies available in more than 100 countries, Baxter’s employees worldwide are now building upon the company’s rich heritage of medical breakthroughs to advance the next generation of transformative healthcare innovations. To learn more, visit www.baxter.com and follow us on Twitter, LinkedIn and Facebook.
Forward-Looking Statements
This release includes forward-looking statements concerning the launch of Progressa+, including potential benefits associated with the use of Progressa+ and anticipated timing of future market launches. The statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those in the forward-looking statements: demand for and market acceptance for new and existing products; product development risks; inability to create additional production capacity in a timely manner or the occurrence of other manufacturing or supply difficulties (including as a result of natural disasters, public health crises and epidemics/pandemics, regulatory actions or otherwise); satisfaction of regulatory and other requirements (including obtaining and maintaining required regulatory approvals); actions of regulatory bodies and other governmental authorities; product quality, manufacturing or supply, or patient safety issues; changes in law and regulations; and other risks identified in Baxter's most recent filing on Form 10-K and Form 10-Q and other SEC filings, all of which are available on Baxter's website. Baxter does not undertake to update its forward-looking statements.
Baxter and Hillrom are registered trademarks of Baxter International Inc. or its subsidiaries.