Baxter and COSMED Announce Partnership to Bring Next-Generation Indirect Calorimetry Device, Q-NRG+, to Support Clinical Nutrition Globally
Non-invasive metabolic monitor accurately measures patient energy expenditure
Designed to help clinicians optimize nutrition therapy in support of improved patient outcomes
Portable and flexible design can be used with intubated and spontaneously breathing patients
DEERFIELD, Ill. and ROME -
Baxter International Inc. (NYSE: BAX), a global leader in clinical nutrition, and COSMED srl, a worldwide leader in the design of metabolic systems for clinical and human performance applications, today announced an agreement to commercialize Q-NRG+, a metabolic monitoring device utilizing indirect calorimetry technology. The agreement provides that Baxter will bring Q-NRG+ to 18 key countries around the world with potential for further expansion. Q-NRG+ is expected to be available from Baxter in September as part of a phased launch in select European countries, Canada and Australia, with launches in additional markets pending future regulatory approval. Additional terms of the agreement were not disclosed.
Indirect calorimetry (IC) is the gold standard1 to accurately measure resting energy expenditure (REE)—a patient’s calorie needs while at rest—and is recommended in guidelines from nutrition societies around the world, including the European Society for Clinical Nutrition and Metabolism (ESPEN)2 and American Society for Parenteral and Enteral Nutrition (ASPEN).3
“For critically ill patients, energy needs can vary dramatically, and traditional ways of predicting these needs may result in under-feeding or over-feeding patients,”4 said Jorge Vasseur, general manager of Baxter’s Clinical Nutrition business. “The introduction of Q-NRG+ represents the next generation of IC technology, enabling individualized metabolic measurements and helping clinicians optimize nutrition therapy in support of improved outcomes for patients.”
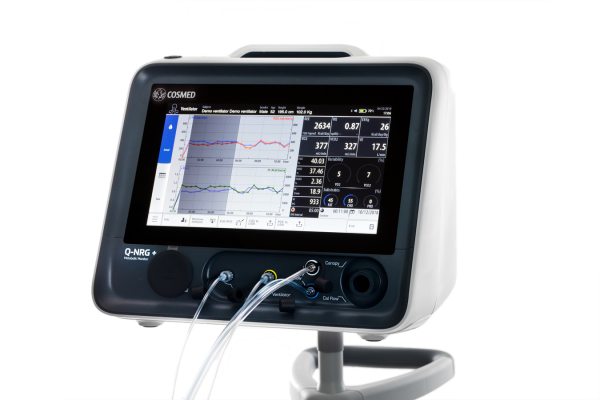
Q-NRG+ is designed to address barriers to rapidly and accurately measure a patient’s REE. Clinicians can use Q-NRG+ to determine energy targets and prescribe and administer the appropriate nutrition therapy for their patients, which may include parenteral nutrition (intravenous feeding). To help ensure patients are prescribed optimal nutrition amounts over the course of their hospital stay, clinicians can also use Q-NRG+ to regularly re-assess energy needs and adjust clinical nutrition prescriptions as necessary.
Q-NRG+ is designed to be flexible, portable and easy to use by clinicians. The device requires minimal warm-up and calibration time and can deliver readings in as few as five minutes. Results are displayed in real-time in a user-friendly “dashboard” on the device’s screen.
“Indirect calorimetry is a validated and well-known technology for measuring energy expenditure in clinical settings, and Q-NRG+ was designed from the ground up to make this important diagnostic tool more accessible and practical for regular use,” said Marco Brugnoli, CEO, COSMED srl. “We look forward to working with our partners at Baxter to bring Q-NRG+ to clinicians and patients around the world.”
About Baxter
Every day, millions of patients and caregivers rely on Baxter’s leading portfolio of critical care, nutrition, renal, hospital and surgical products. For more than 85 years, we’ve been operating at the critical intersection where innovations that save and sustain lives meet the healthcare providers that make it happen. With products, technologies and therapies available in more than 100 countries, Baxter’s employees worldwide are now building upon the company’s rich heritage of medical breakthroughs to advance the next generation of transformative healthcare innovations. To learn more, visit www.baxter.com and follow us on Twitter, LinkedIn and Facebook.
About COSMED
COSMED, established in 1980, is a privately-owned company that designs and manufactures cardio pulmonary and metabolic diagnostic equipment. COSMED solutions address needs of the healthcare, academic and industry markets to assess human metabolism, exercise physiology, pulmonary function and body composition for clinical and research purposes. COSMED products include a full range of spirometers, indirect calorimetry, cardio pulmonary exercise testing and body composition systems including software. COSMED headquarters are located in Rome, Italy with direct operations in Australia, France, Germany, Hong Kong, Netherlands, Switzerland, UK and United States, and a network of business partners covering more than 80 countries. Visit www.cosmed.com to know more about COSMED.
This release includes forward-looking statements concerning Baxter and Q-NRG+, including Q-NRG+ indications, use, effectiveness and risks and expectations with regard to its availability worldwide. The statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those in the forward-looking statements: satisfaction of regulatory and other requirements; actions of regulatory bodies and other governmental authorities; product quality, manufacturing or supply issues; patient safety issues; changes in law and regulations; and other risks identified in Baxter's most recent filing on Form 10-K and other SEC filings, all of which are available on Baxter's website. Baxter does not undertake to update its forward-looking statements.
Baxter is a trademark of Baxter International Inc. Q-NRG is a trademark of COSMED.
1 Frankenfield D, Ashcraft C. Estimating Energy Needs in Nutrition Support Patients. Journal of Parenteral and Enteral Nutrition. 35(5). 2011. 563-570.
2 Singer P, Blaser AR, Berger M, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clinical Nutrition. 38. 2019. 48-79.
3 McClave S, Taylor B, Martindale R, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Journal of Parenteral and Enteral Nutrition. 40(2). 2016. 159-211.
4 Zusman O, Kagan I, Bendavid I, et al. Predictive equations versus measured energy expenditure by indirect calorimetry: A retrospective validation. Clinical Nutrition. June 2019. 38 (3) 1206-1210.